All Articles

Robitussin Real-World Evidence: Relief and Quality of Life Improvements from Day 1
Robitussin conducted the first Real-World Evidence (RWE) study in the cough and cold category—to see how our products help people feel better and live better, starting from Day 1.

featured article
What Causes a Scratchy Throat?
Learn what causes a dry, scratchy throat and how you can get relief from your symptoms.

featured article
What to Do if You Can't Stop Coughing in Class
Explore ways to deal with a cough that comes on quickly when you’re at school.

featured article
What Does DM Mean in Cough Medicine?
Learn what DM refers to in cough syrups, and see why cough medicines with this common notation are helpful to alleviate multiple symptoms.

featured article
Cold vs. Flu
Know your symptoms

featured article
Cough Heroes
Dextromethorphan and Guaifenesin

featured article
Mucus vs. Phlegm
What you should know

Why One Side of Your Nose Can Get Clogged
Learn why only one of your nostrils feels blocked and how you can clear it.

Teach Your Kids to Cough Safely
Help your children prevent the spread of infectious diseases.

Sick Etiquette
Germs never take a sick day. Check out the following do’s and don’ts from etiquette expert Diane Gottsman, and help limit the spread of germs.
Cold & Flu Symptom Awareness
A new survey conducted online by Harris Poll on behalf of Robitussin found that while consumers are aware of sick etiquette, they have some work to do when it comes to proving it.

Understanding Coughs
Learn how to take care of and control a cough.

The Cold Hard Cough Facts
The common cold and cough affects millions of people each year.

How Cold Medicine Works
Find relief for common cold symptoms.

Understanding what your cough is telling you
The two types of cough — wet cough and dry cough — are associated with different conditions.

Soothing Liquids
When treating a cough with cough syrups, it is important to consider the active ingredients as well as both the taste and form in which the medication is taken.
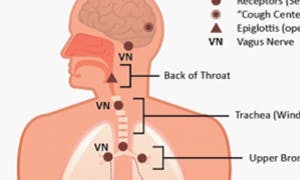
Anatomy of the Cough
Even though a cough is unpleasant, annoying and disruptive to everyday life, it actually serves as one of the body’s defense mechanisms.

Proper Dosing for Children
Learn how to determine dosing and medications for your little ones.

Cold and Flu Fast Facts
Get quick and helpful information about colds and flu.

Why Medicine?
Find out what types of medicines can help ease your cold symptoms.

8 Indoor Activities for Kids
Whether your little ones are at home because of bad weather, school closures or other circumstances, keeping them occupied can feel daunting.

5 Staycation Ideas
Vacation should be a time for people to unwind and relax from their day-to-day responsibilities of work or school.

Wash Your Hands Songs – Tunes for Effective Washing
The simple act of washing your hands is vital for preventing the spread of germs, diseases, and illnesses from one person to another.

How to Cover Your Cough – Tips for Covering Your Cough and Sneeze
Covering your cough and sneeze is one of the easiest ways prevent the spread of germs.

Immune System Booster Foods & Ingredients to Fight Illness
Whether it’s cough and cold season or you’re trying to avoid a bug that’s going around, immune system booster foods can be a helpful addition to your diet.

How to Stay Healthy While Traveling
Traveling for work or play often comes with the risk of picking up a bug.

Why Does my Toddler Cough at Night?
Discover what may be making your toddler cough at night. Robitussin has some tips to help them feel better so you can both get some restful sleep.

How to Help Relieve Chest Congestion Using Home Remedies
Chest congestion can be tough to get rid of if you don’t know what’s causing it and how to get the mucus out.

What Causes Excess Mucus?
Discover what causes excess mucus when you’re feeling under the weather. Robitussin can relieve cold or flu symptoms.

Green Phlegm: What You Need to Know
Understand what causes green phlegm and how you can ease your coughing with this overview from Robitussin. Coughing up green phlegm isn’t necessarily cause for alarm.

Chest Congestion Causes & Symptoms
Understand what causes chest congestion, how to recognize the symptoms of chest congestion, and remedies to ease chest congestion with this Robitussin overview.

Runny Nose vs. Stuffy Nose: What’s the Difference?
Discover the differences between a runny nose and a stuffy nose. Robitussin can help you treat these symptoms of a cold or flu so you can breathe easily.

How to Loosen Chest Congestion When You’re Sick
Let Robitussin help you find out how to loosen chest congestion. Discover helpful tips on breaking up chest congestion and easing symptoms.

Why Do I Cough at Night
Learn from Robitussin why you cough at night and what causes your cough to worsen. Discover ways to relieve your nighttime cough and get a good night’s sleep.

How to Relieve a Dry Cough
Learn how to relieve a dry cough with Robitussin. Find out what can cause a dry cough and get tips and tricks on getting dry cough relief.

Stuffy Nose at Night: Why Do I Get Congested When Lying Down?
Learn what causes you to have a stuffy nose at night with this article from Robitussin. Discover how to relieve the nasal congestion before you go to bed.

What Is Catarrh?
Discover what catarrh is and learn the meaning of this unusual word. Robitussin can help you find effective relief for your uncomfortable symptoms.

What Is Post-Nasal Drip?
Learn what post-nasal drip is and how to treat it with Robitussin. Post-nasal drip happens when you swallow mucus unconsciously and can lead to discomfort.

Why Does My Body Ache?
Learn about body aches and what causes them with this article from Robitussin. See why you get body aches when you’re sick and find tips on helping with pain.

How to Break a Fever at Home
Start feeling better and learn how to break a fever at home. When you’re sick at home with the flu and a fever, Robitussin can help you feel like yourself again.

What Does Your Snot Color Mean?
Learn about changes in your snot color with this article from Robitussin. Discover what causes excessive nasal mucus and what the color of your snot means.

Sinus vs. Nasal Congestion
Learn about sinus vs. nasal congestion with this article from Robitussin. Discover what sinus congestion is and how it differs from nasal congestion.

Can You Take Zinc for Colds?
Find out if you should use zinc for colds with this article from Robitussin. Learn more about zinc supplements for colds and the risks with taking them.

Is It A Cold or Allergies?
Learn how to tell the difference between allergies and a cold with this guide from Robitussin. Find out if your symptoms are caused by a cold or allergies.

Why Am I Coughing Up Mucus?
Stop coughing up mucus and clear your chest congestion with a little help from Robitussin. Learn how to get relief from your symptoms and start feeling better.

Does a Humidifier Help with Congestion and Cough?
Understand how a humidifier helps with congestion and cough. Robitussin explains how this useful tool can help you breathe easier when you’re feeling sick.

Can Coughing Cause Sore Throat Pain?
Learn how a cough can cause a sore throat and use these tips from Robitussin to relieve sore throat pain.

Treating Your Kid’s Cough and Runny Nose
Find out why your kid has a cough or runny nose, and get tips on finding them relief.

How to Pinpoint the Cause of Your Dry Cough
Learn about the cause of your dry cough with this guide from Robitussin. Talk to your doctor to pinpoint the reason and find treatment that works for you.

What Helps a Sore Throat and Cough?
Find out which remedies can provide you with relief from your sore throat and tickly cough.

What You Need to Know About Dark Yellow Mucus
Learn what causes dark yellow mucus with this primer from Robitussin. The mucus in your body is an essential part of your immune system and keeps you healthy.

What Happens When You Cough?
Find out what a cough is, what makes you cough and how the coughing reflex works.

How to Help a Runny Nose at Night
Learn how to help a runny nose at night with Robitussin. Find out what’s causing your runny nose and get tips to relieve it so you can sleep better.

Why do Fevers Come with Chills?
Understand the connection between fever and chills. Learn what causes chills and find effective home remedies.

How Long Does a Cough Last After the Flu?
Learn how long a cough can last after the flu with Robitussin. Find out what a flu cough looks like and how a cough can affect you while you recover.

Why Does Flu Season Exist?
Learn why the flu season exists, when it starts and how to protect yourself with these tips with these tips.
What Causes Bronchial Irritation?
Alleviate bronchial irritation and bronchitis symptoms. Learn what causes bronchial irritation so you can get relief.

Why Isn't There a Vaccination Against the Common Cold?
Discover why no vaccination has been developed for the common cold.

Understanding Throat Congestion: Dealing with Postnasal Drip
Find out what throat congestion (more commonly known as postnasal drip) is and how to treat it.

How Dextromethorphan Helps Coughs
Learn how dextromethorphan helps to provide relief for cough symptoms from the cold and flu.

How to Get Rid of a Cough Fast
Learn how to treat a cough that comes on quickly.

How to Keep Your Cough to Yourself While Traveling
See how far a cough can travel and how to keep yourself others healthy.